The first FDA-approved device to treat ADHD, explained
Children with attention deficit hyperactivity disorder have a new, nondrug treatment option for managing the condition.
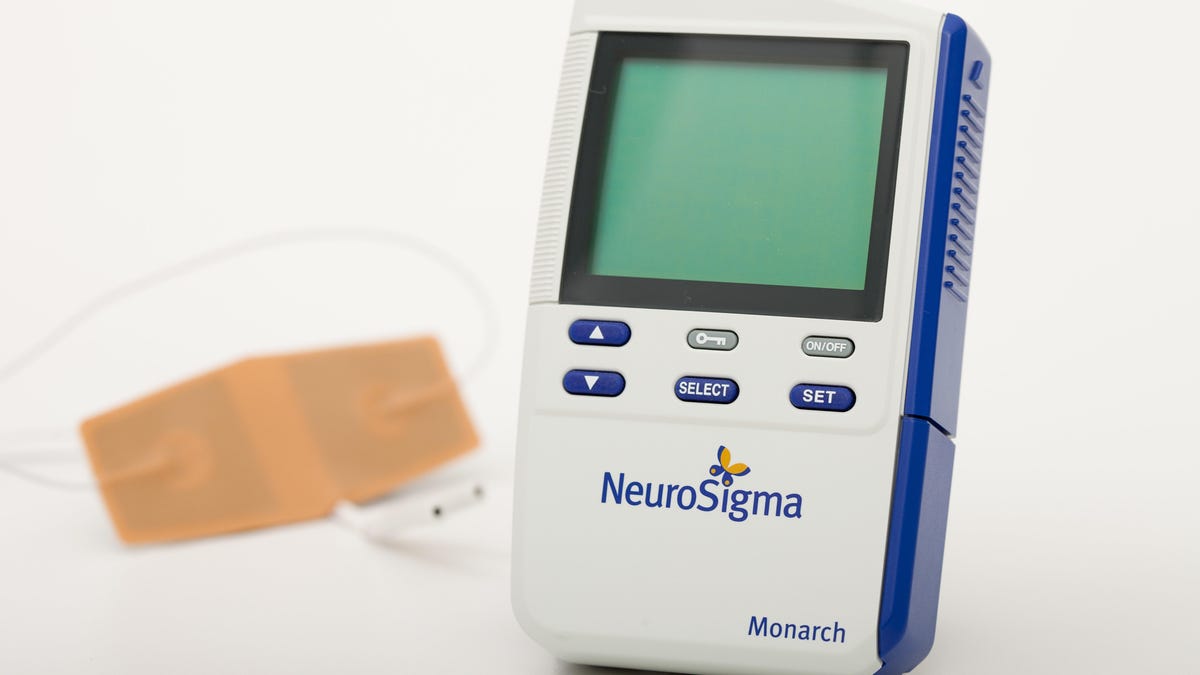
For decades, managing ADHD has meant getting a prescription for Adderall or Ritalin. Now children have another option: the Monarch external Trigeminal Nerve Stimulation (eTNS) system, an ADHD treatment device that works by sending mild electrical stimulation shocks to the nervous system.
The Food and Drug Administration gave the device clearance to be marketed as a treatment for patients between 7 and 12 years old. Here's what you need to know about the Monarch eTNS.
First, what is ADHD and how is it diagnosed?
Attention deficit hyperactivity disorder is a common condition that begins in childhood and is often difficult to manage. Symptoms include inattention, difficulty focusing, impulsivity and high levels of activity.
To be diagnosed with ADHD, a person must exhibit persistent inattention and hyperactivity that's long-lasting and interferes with important parts of life. An example of inattention is failing to pay attention to details on school assignments, while examples of hyperactivity include fidgeting, blurting out or excessive speech and spontaneous bouts of movement, such as jumping or running.
Additionally, several symptoms must have been present before age 12, and several symptoms must be present in more than one location. For example, a child might exhibit inattention and hyperactivity at school and in the car.
What's the Monarch eTNS system?
The Monarch eTNS is a newly released medical device that attaches to the forehead via a patch and small wire. It sits just above the eyebrows and delivers a slight tingling sensation on the skin, an effect of the low-level nerve stimulation.
The Monarch system comes from California-based life sciences company NeuroSigma, which develops treatments and devices for neurological disorders.
How does the Monarch eTNS work?
The electric pulses from this cell phone-size device interact with the trigeminal nerve, which then sends therapeutic signals to the parts of the brain thought to be responsible for ADHD symptoms.
"This new device offers a safe, nondrug option for treatment of ADHD in pediatric patients through the use of mild nerve stimulation, a first of its kind," Carlos Pena, director of the FDA's Division of Neurological and Physical Medicine Devices, said in a statement.
The FDA approved Monarch eTNS for use in managing ADHD symptoms after reviewing a double-blind, randomized, controlled trial of 62 children with moderate-to-severe ADHD. Details of the trial were published in the Journal of the American Academy of Child and Adolescent Psychiatry in January. In the study, 32 children with ADHD used the Monarch eTNS device every night, and 30 children with ADHD used a placebo device.
The children who used the real device over the four-week trial period exhibited a greater reduction in their symptoms than the children who used the placebo.
What are the side effects and is it safe to use?
According to the 2019 study's authors, a big advantage of the Monarch eTNS system is the apparent lack of any serious health risks. The study notes that side effects include drowsiness, an increase in appetite, trouble sleeping, fatigue, headaches and teeth-clenching.
Even though it's marketed as extremely safe, Monarch eTNS is intended for use only under the supervision of a caretaker. Also, treatment with the Monarch system takes about a month before symptoms visibly improve. It's recommended that children who use Monarch eTNS check in with their doctor after four weeks to evaluate treatment effects.
Children who use the following shouldn't use Monarch eTNS:
- A pacemaker or other implanted device.
- An insulin pump or other body-worn medical device.
- Prescription ADHD medications such as Adderall or Ritalin.
How can I get the Monarch eTNS?
Monarch eTNS is already available in Canada, Australia and Europe as a treatment for epilepsy and depression. There's no US release date yet.
Monarch eTNS is available only with a prescription, which first requires an ADHD diagnosis.
Additionally, children can use Monarch eTNS only if they aren't currently using a prescription medication to manage ADHD symptoms. It's also recommended that children with pacemakers, insulin pumps or other bodily devices don't use the Monarch system.
What does this mean for future ADHD treatments?
The marketing approval of the Monarch eTNS from the FDA sets a precedent for similar devices to follow.
"This action creates a new regulatory classification, which means that subsequent devices of the same type with the same intended use may go through the FDA's 510(k) premarket process, whereby devices can obtain marketing authorization by demonstrating substantial equivalence to a predicate device," the FDA wrote in its statement.
This precedent will allow similar devices to move through the FDA's approval process much faster.
The information contained in this article is for educational and informational purposes only and isn't intended as health or medical advice. Always consult a physician or other qualified health provider regarding any questions you may have about a medical condition or health objectives.

