FDA to Loosen Blood Donation Guidelines That Restricted Gay, Bisexual Men
The new guidance will focus on recent sexual history and individual health assessments.
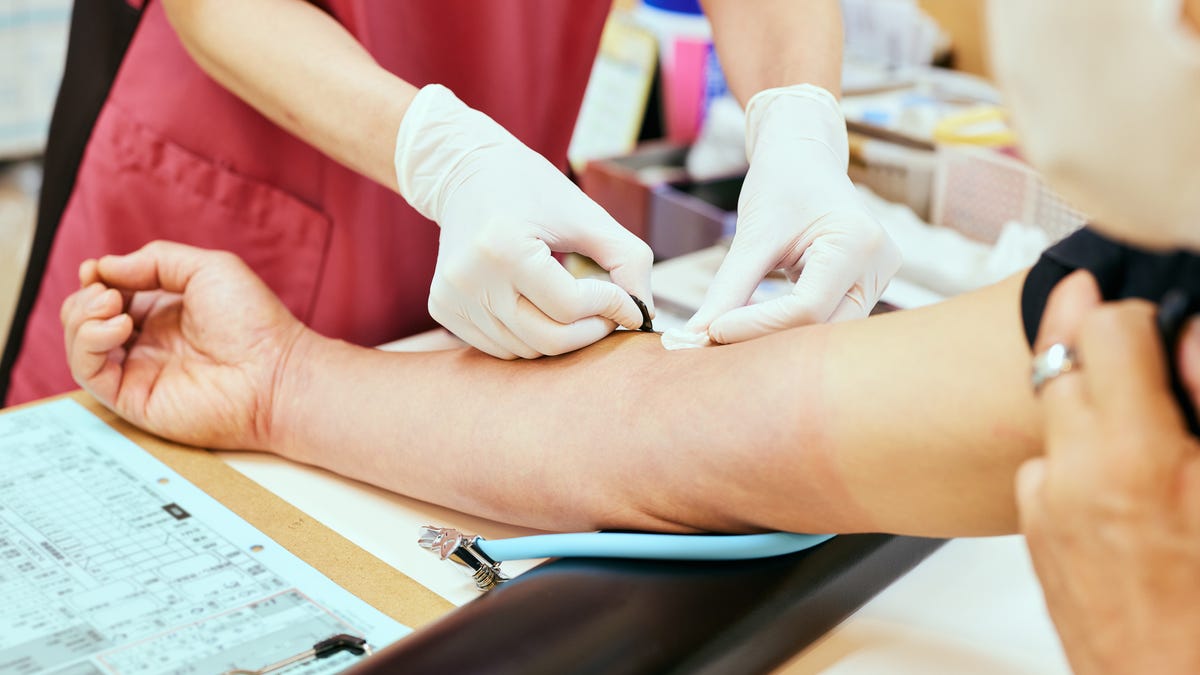
The US Food and Drug Administration on Friday announced changes to rules that have long restricted many gay and bisexual men from giving blood. The new guidance, which has yet to go into effect, will instead focus on individual health screenings and take into account the recent sexual history of all donors.
As it stands now, blood donation rules ban any man who has sex with men (MSM) from donating if they've had sexual contact in the last three months, including among long-term partners. The same waiting period to give blood also applies to anyone who has sex with MSM. Many in the activist and medical communities have spoken out against these rules as discriminatory and outdated because they single out MSM, without questioning the sexual behavior of others.
According to the new proposal, which should be finalized by the FDA in the coming months, MSM and their partners would be able to donate blood without any waiting period. However, there will still be restrictions on those who have had anal sex with new or multiple partners within the last three months. The donation restrictions based on other criteria, such as recent injection drug use and having sex in exchange for money, will also remain in effect.
The proposal comes as the US faces a blood supply problem, and is in line with blood donation guidelines in other countries, such as Canada and the UK. The FDA said that its draft recommendations are based on a review of available information, including data from countries that already implement the guidelines.
A safe blood supply and donation process is "paramount" to the FDA, the agency's commissioner, Dr. Robert M. Califf, said in a news release, and maintaining it is important.
"This proposal for an individual risk assessment, regardless of gender or sexual orientation, will enable us to continue using the best science to do so," Califf said.
Friday's announcement by the FDA is the latest move to loosen a decades-long practice of stopping men who have sex with men from giving blood. Until 2015, gay and bisexual men weren't allowed to donate blood at all. In 2020, the FDA shortened its deferral period for men who have sex with men, changing the requirement from waiting 12 months since the last sexual contact to three months.
The blood donation rules and bans, largely impacting gay men but also restricting others, including people who've gotten tattoos or inject drugs, are based on the assessment of who's more at risk for contracting human immunodeficiency virus (HIV). The lifetime blood donation restriction on men who have sex with men that expired in 2015 had been created in the 1980s during the HIV/AIDS crisis, which disproportionately impacted gay and bisexual men. HIV spreads more easily through anal sex, which is why it remains a focus in the new donation guidance.
In the decades since the restrictions were put in place, the US has moved ahead in HIV awareness and prevention, and advancements in science and medical testing have extended to blood donation clinics. Donor blood is already tested for infectious diseases, including HIV and hepatitis B and C.
"This proposed blood donation policy moves the country toward what LGBTQ+ advocates and medical experts have been saying for years," Kelley Robinson, president of the Human Rights Campaign, said in a press release Friday. "That a science-based, individualized risk assessment is the best, most equitable way to ensure safety of the blood supply while reducing unnecessary discrimination against gay, bisexual, and other men who have sex with men."
According to the new proposed guidelines, time-based restrictions for those taking PrEP (an HIV drug that helps prevent infection pre-exposure) and PEP (a drug taken post-exposure) will still be in effect, the FDA said. People taking PrEP and PEP should continue their medication and not stop in order to donate blood, the HRC said.

