Coronavirus plasma treatment approved for emergency use, but questions remain
The FDA says the treatment -- which utilizes blood from infected individuals -- may lessen the severity of the disease, but experts suggest further research is needed.
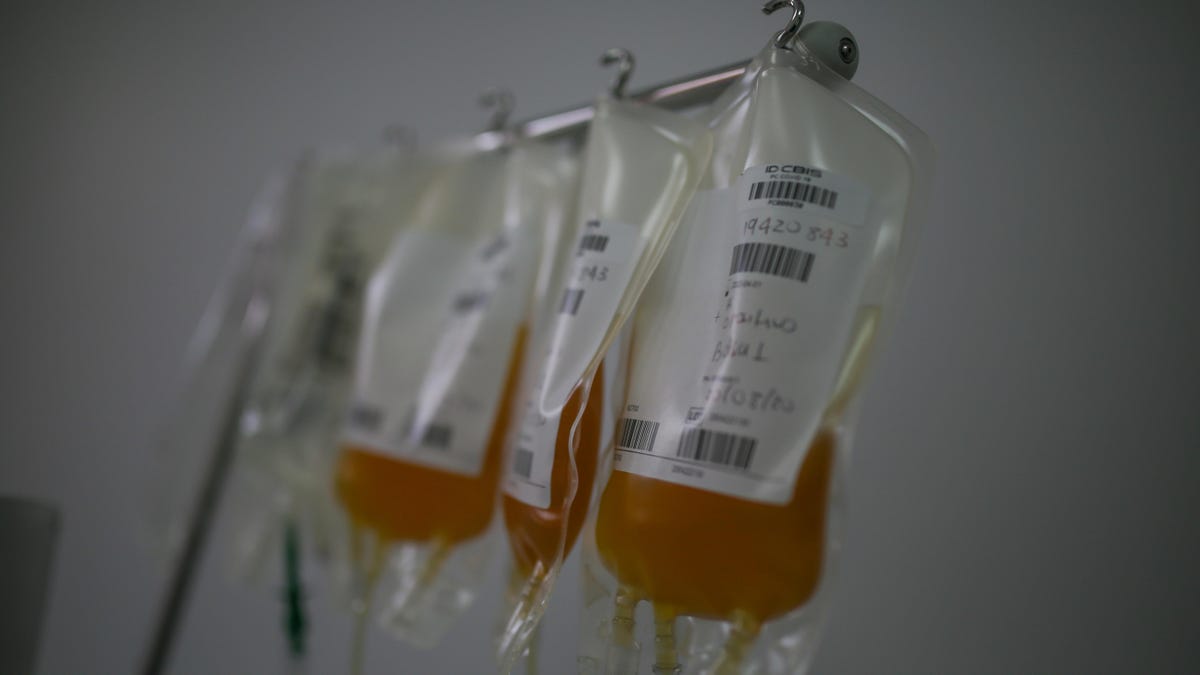
Convalescent plasma is an experimental COVID-19 treatment that has shown some promise in battling severe illness.
The U.S. Food and Drug Administration has granted an emergency use authorization for convalescent plasma, an experimental treatment option which utilizes blood from patients who have recovered from COVID-19, opening up access as clinical trials of the treatment continue.
Alex Azar, the U.S. health and human services secretary, said during a White House press briefing on Sunday that the treatment has been delivered to more than 70,000 American patients so far. The treatment, according to the FDA's evaluation, "may be effective in lessening the severity or shortening the length of COVID-19 illness in some hospitalized patients."
This isn't the first time such a therapy would be used; previous outbreaks of SARS, MERS and the H1N1 influenza pandemic all saw the use of convalescent plasma to treat patients. In fact, convalescent plasma was even used in the 1918 influenza pandemic.
However, it's unclear how effective it is in COVID-19 and clinical trials across the world are evaluating its use, though it is considered safe. "At this time, there is good evidence of safety," says Zoe McQuilten, a haematologist at Monash Health in Australia.
When patients are infected by the virus which causes COVID-19, SARS-CoV-2, their bodies mount an immune response, building tiny Y-shaped proteins known as antibodies. Antibodies then traverse the body in the blood. Scientists can draw blood from recovered patients and separate out the plasma, a yellowish portion of the blood which contains the antibodies. The plasma can then be transfused into a sick patient, where it may help fight the infection.
"The data from studies conducted this year shows that plasma from patients who've recovered from COVID-19 has the potential to help treat those who are suffering from the effects of getting this terrible virus," said Stephen Hahn, the FDA commissioner.
Despite Hahn's confidence, the jury is still out on how effective convalescent plasma might be in battling the pandemic.
"Proof of effectiveness can come only from well-designed randomised controlled trials in which patients given the treatment are compared with patients who could have received the treatment but didn't receive it," notes McQuilten. "Such trials have commenced but have not yet been completed. Until such trials are completed and reported, the effectiveness of convalescent plasma remains uncertain."
The treatment faces two major hurdles.
First, you need a significant number of sick patients to donate blood and doctors access is contingent on these donations. Not everyone can donate their plasma. "Quite a lot of people who recover from COVID-19 have underlying health issues or are disqualified from being donors for other reasons," notes Ian Gust, an immunologist at the University of Melbourne, Australia.
Second and more importantly, results from clinical trials haven't found any clear-cut advantages in using the treatment. The National Institutes of Health database currently lists dozens of clinical trials for convalescent plasma and COVID-19 but there is a lack of robust data from rigorous study.
A report by Chinese scientists published in journal The Lancet Infectious Diseases in February suggested the treatment option may be viable in battling SARS-CoV-2 and anecdotal evidence from China has demonstrated some success, with 91 of 245 patients in a trial showing improvement, according to Xinhua.
A larger study, performed by the Mayo Clinic in June, demonstrated the therapy was safe in a study of around 20,000 patients. Serious adverse events were seen in less than one perfect of cases.
In June, a paper published in the Journal of the American Medical Association, did not find any improvements in patients using the treatment. However, the study was based in China as health authorities began to gain control over the outbreak, so it was terminated early and may not have been adequately powered to find any differences between patients receiving and not receiving the treatment.
When Australia announced it would begin convalescent plasma trials on July 30, Steven Tong, a Royal Melbourne Hospital infectious diseases clinician, noted it was an "attractive" concept but clinical trials were required to "demonstrate efficacy for patients with COVID-19."

