Robotic exoskeleton for paraplegics approved for market
A robotic exosuit that will help paraplegics stand and walk is the second to be approved for clinical and personal use by the FDA.
After 10 years in development and a clinical trial that involved some 1,200 patient sessions, the US Food and Drug Administration has approved a robotic exoskeleton designed by a team of researchers at Vanderbilt University.
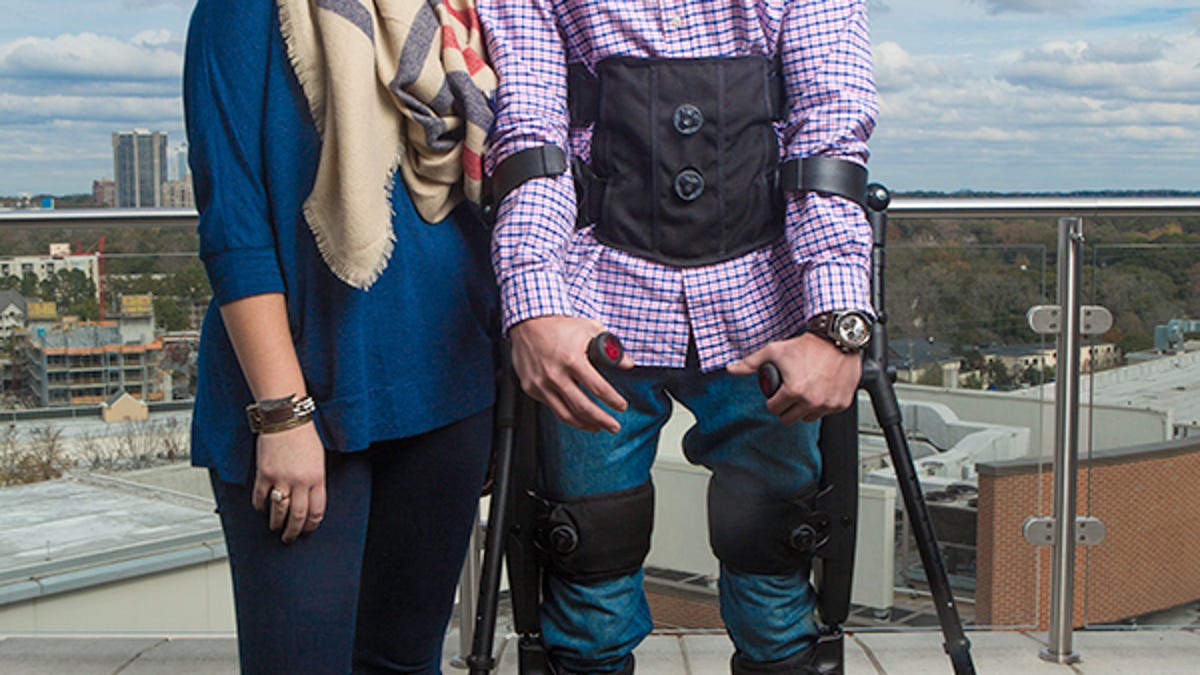
The Indego exoskeleton is the second exoskeleton in America to receive FDA approval for clinical and personal use, and will allow people who have been paralysed below the waist to stand up and walk around. You can view an online brochure here (PDF).
"You can think of our exoskeleton as a Segway with legs," explained Michael Goldfarb, the professor of mechanical engineering who led the project, in a statement.
"If the person wearing it leans forward, he moves forward. If he leans back and holds that position for a few seconds, he sits down. When he is sitting down, if he leans forward and holds that position for a few seconds, then he stands up."
The exoskeleton is a rigid framework that fits around the patient's legs, with powered joints at hip and knee. A wide belt straps around the torso to keep the exoskeleton secure, and crutches or a walking frame helps the wearer keep their balance. It's powered by a rechargeable battery that can be taken out and replaced as need arises.
The Indego exoskeleton will automatically adjust the amount of robotic assistance to the individual. If the wearer has some mobility, the exoskeleton will supply less assistance, which not only preserves the battery life, it will allow the wearer to use their own muscles. For wearers with no mobility, the Indego will operate at full power.
Moreover, the Indego integrates a rehabilitation technology called functional electrical stimulation. This uses gentle electrical currents to activate nerves, and has been proven beneficial. In the case of the Indego, small electrical pulses cause the muscles to expand and contract, increasing strength in patients with some mobility, or improving circulation and reducing muscle atrophy in patients with complete paraplegia.
Initially, the exoskeleton, which has been available in Europe since November 2015, will cost $80,000 per unit. However, the team hopes next to get Indego approved for insurance reimbursement, which will make it much more accessible.
"I'm really glad," said Goldfarb said. "It is particularly gratifying because it is the first thing that has come out of my lab that has become a product that people can purchase, which hopefully will make a significant improvement in their quality of life."