Man with kidney disease first in U.S. to get bioengineered vein
In a first-of-its kind procedure, surgeons implant the blood vessel into the arm of a 62-year-old Virginia man with renal failure.
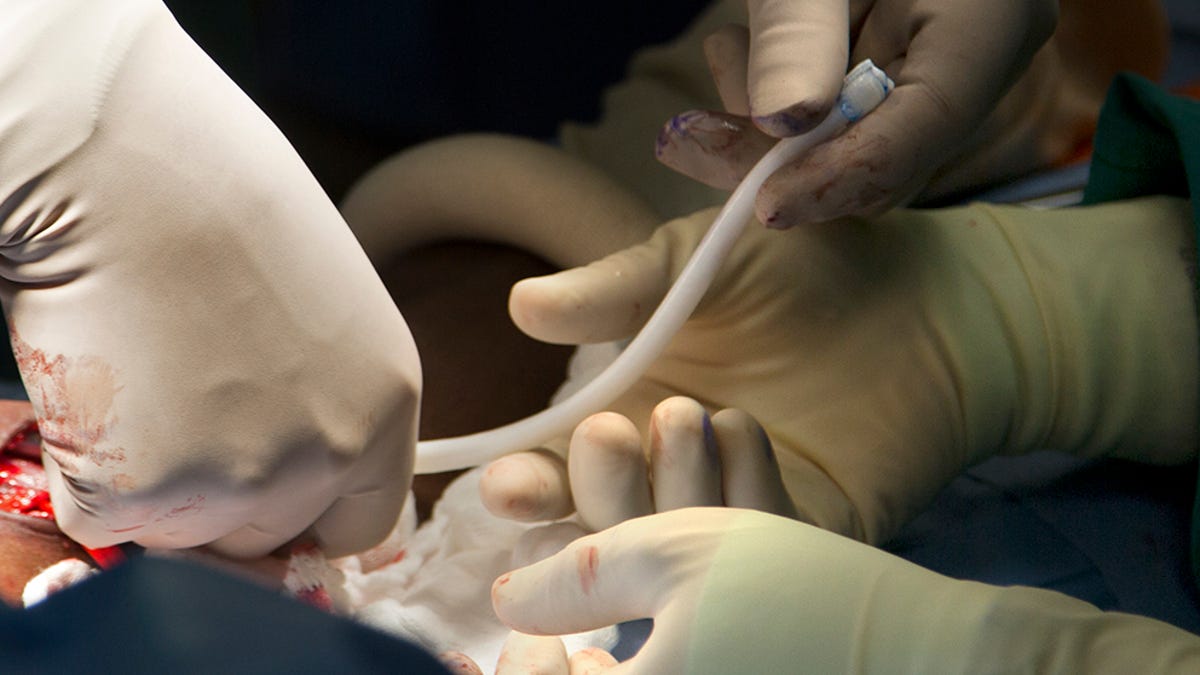
For the first time in the U.S., surgeons have successfully transplanted a bioengineered blood vessel into the arm of a patient -- a possible stepping stone toward more complex human-engineered organs such as livers or eyes, and potentially a more immediate boon for kidney dialysis patients and perhaps even people with heart disease.
The surgery represents a major milestone for tissue engineering: The bioengineered blood vessel can be stored relatively easily and donated universally (unlike veins harvested from a patient's own body and therefore specific to that body). Also, it's human-cell-based, with no biological properties that can cause organ rejection.
"We hope this sets the groundwork for how these things can be grown, how they can incorporate into the host, and how they can avoid being rejected immunologically," Jeffrey H. Lawson, a vascular surgeon and biologist at Duke Medicine who helped develop the technology and performed the implantation, said in a statement. "A blood vessel is really an organ -- it's complex tissue. We start with this, and one day we may be able to engineer a liver or a kidney or an eye."
The U.S. surgery comes on the heels of an early clinical trial in Poland in December, which followed 15 years of research. Since the FDA approved the phase 1 trial that will ultimately involve 20 kidney dialysis patients in the U.S., researchers at Duke who helped create the blood vessel are serving as study leaders before submitting the results to a safety review.
If the bioengineered veins prove effective and safe for these first 20 hemodialysis patients, it will be good news for the more than 350,000 people in the U.S. who require hemodialysis and currently deal with issues that range from clotting when given synthetic grafts to additional procedures and risk of infection when harvesting veins from the patient's body. And researchers say they ultimately hope to translate what they learn here to developing a readily available and durable graft for heart bypass surgeries (performed on some 400,000 people in the U.S. annually) and to treat blocked blood vessels in limbs.
To build the vein, which was implanted into the arm of a 62-year-old Virginia man with kidney disease, engineers cultivated donated human cells on a tubular scaffold to form a vessel-like shape, which they then scrubbed clean of qualities that can trigger immune responses. This turned out to work better in preclinical testing than other synthetic and animal-based implants had.
Niklason and Laura Niklason, co-founder of spin-off Humacyte and a former faculty member at Duke who is now at Yale, were able to perfect the technology in animal models before moving to human veins. In animals, the implanted vein grafts ultimately adopted the cellular properties of a blood vessel, so not only were they not rejected by the host, but they actually became indistinguishable from living tissue as cells grew into the implant.
To accomplish this, the researchers used biodegradable mesh as scaffolding, which they could manipulate easily into the shape of a blood vessel of whatever length or width they wanted. Then they seeded the mesh with smooth muscle cells, and the mesh eventually dissolved as the cells grew in a medium of amino acids, vitamins, and other nutrients. By introducing a pulsating force during this growth stage, the researchers were able to pump the nutrients through the tube in a heartbeat rhythm -- as close to the real thing as seems possible. A couple of months later, they were looking at a lifelike vein.
The researchers say that the main reason they're not harvesting a person's own cells to seed the scaffolding, which would lower the risk of tissue rejection, is because growing these personalized veins took too long, thus ruling out mass production. Their current matrix is washed in a "special solution" that rinses out cellular properties that could cause rejection, so the end result is, in Niklason's words, "a nonliving, immunologically silent graft that can be stored on the shelf and used in patients whenever they need it."
Just one day after the two-hour procedure at Duke, it's too early to tell whether the Virginia man's body will incorporate the bioengineered vein without issues, but these next few months will provide a lot of insights into whether the vein becomes "functionally alive," as Lawson puts it, which would ultimately be good news for hundreds of thousands of patients worldwide.

