FDA approves first digital drug to track if you take your meds
The digital ingestion tracking system is the first to be approved in the United States. Say ahh.
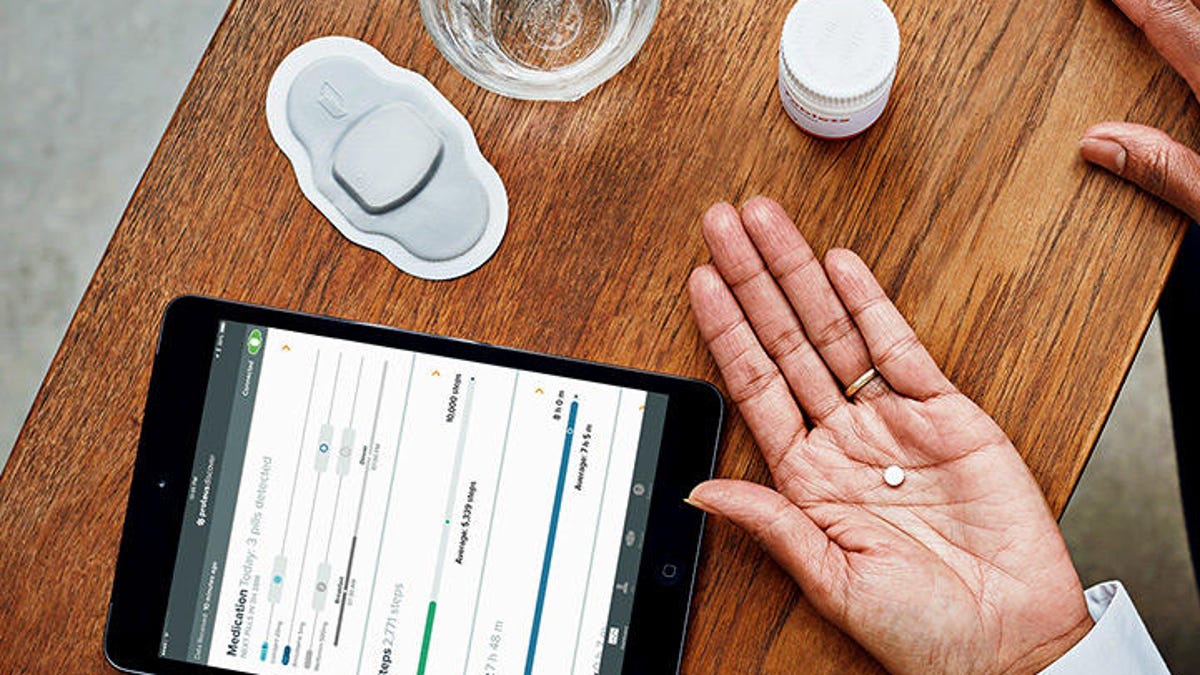
The sensor, embedded in the pill, sends information to the wearable patch that can be accessed on a phone or tablet.
Following the doctor's orders might soon be unavoidable advice.
The US Food and Drug Administration on Monday approved the first drug in the US with a digital ingestion tracking system.
Abilify MyCite, an aripiprazole tablet embedded with an ingestible sensor, uses digital tracking to record whether the medication was taken. The tablet has been approved for the treatment of schizophrenia, acute treatment of manic and mixed episodes associated with bipolar I disorder, and for use as an add-on treatment for depression in adults, the FDA said.
The pill's sensor sends a message to a wearable patch that transmits the information to an app, allowing patients to track the medication's ingestion on their phone. Patients can also let their doctor or carer view the information through a portal online.
Abilify MyCite's sensor has been around since 2012, developed by Proteus Digital Health. In 2016, British Airways got in on the digital drug game, patenting a sensor-packed smart pill that measures your temperature, stomach acidity and more to help fight jet lag.
Rebooting the Reef: CNET dives deep into how tech can help save Australia's Great Barrier Reef.
The Smartest Stuff: Innovators are thinking up new ways to make you, and the things around you, smarter.

