Oxford COVID-19 vaccine gets approval for emergency use in UK
The vaccine developed by Oxford University and AstraZeneca will begin to be administered next week.
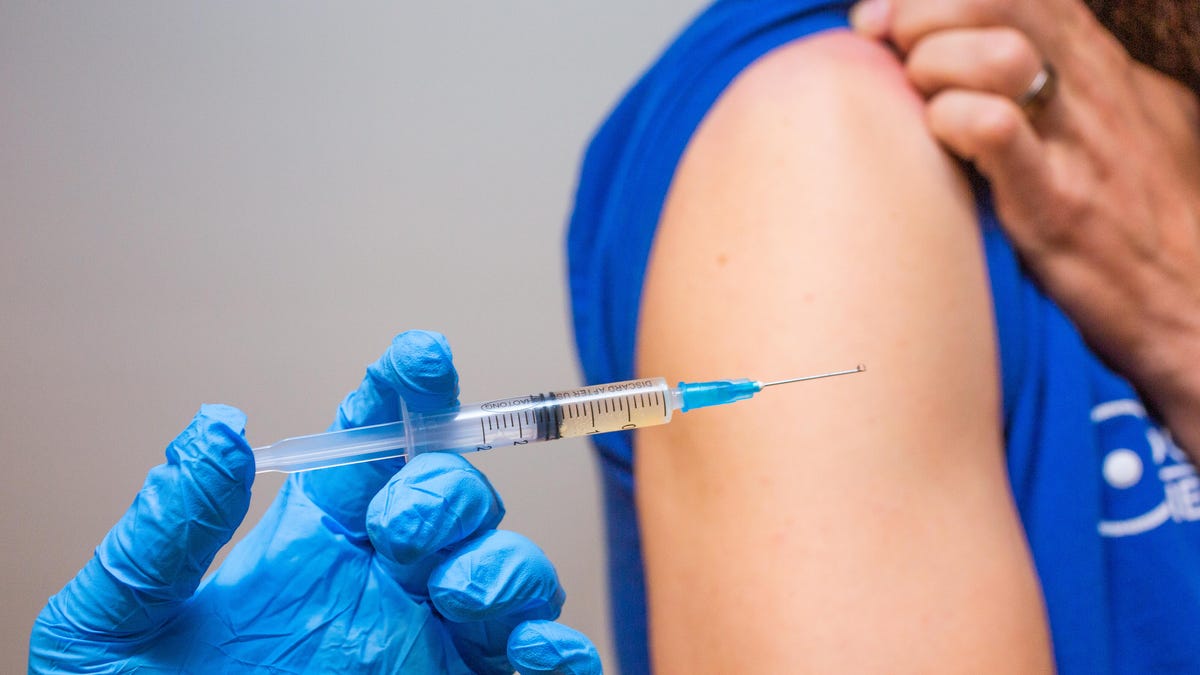
The first Oxford University/AstraZeneca COVID-19 vaccination will apparently happen next Monday.
The Oxford University and AstraZeneca COVID-19 vaccine was approved for emergency supply in the UK, the country's government said Wednesday. This follows the Moderna and Pfizer vaccines winning approval last month.
Like the other two, the Oxford vaccine will require two doses. In this case, the second shot should be administered between four and 12 weeks after the first.
The first person will get the Oxford vaccine on Jan. 4, UK Health Secretary Matt Hancock told the BBC, and the UK ordered enough to vaccinate the country's entire adult population.
"This is a moment to celebrate British innovation -- not only are we responsible for discovering the first treatment to reduce mortality for COVID-19, this vaccine will be made available to some of the poorest regions of the world at a low cost, helping protect countless people from this awful disease," Hancock said in a statement.
Read more: COVID-19 vaccine facts: Hidden costs, when you can get vaccinated
Unlike the Pfizer vaccine, which has to be kept in ultracold temperatures, the Oxford vaccine can be stored in "normal refrigerated conditions," AstraZeneca said. (The Moderna vaccine can also be refrigerated.)
The approval comes after a "highly contagious" new coronavirus strain was detected in the UK last month, sending the country into a strict lockdown over the holiday season. This first US case of this new variant was discovered Tuesday, in Colorado.
The European Medicines Agency won't assess the Oxford vaccine until January at the earliest because it hasn't received an application from AstraZeneca, while US regulators are waiting for a late-stage trial to end, according to the Financial Times.
"EMA is currently assessing data on this vaccine as part of a rolling review, which started on Oct. 1 2020," an EMA spokesperson said in a statement emailed to CNET. "EMA's human medicines committee has assessed data from laboratory studies (nonclinical data), and is currently assessing data on the vaccine's quality (on its ingredients and the way it is manufactured) as well as evidence on its efficacy and safety coming from several ongoing clinical trials."
The US Food and Drug Administration declined to comment on the Oxford vaccine specifically, but a spokesperson noted via email that it's "committed to providing the same expeditious, thorough and transparent review" process it applied to the Pfizer and Moderna vaccines to any future Emergency Use Authorization requests.

