There's Finally an Ample Supply of Paxlovid. So Why Aren't More People Taking the COVID Antiviral Pill?
Dr. Anthony Fauci says Pfizer's antiviral COVID pill "is being underutilized."
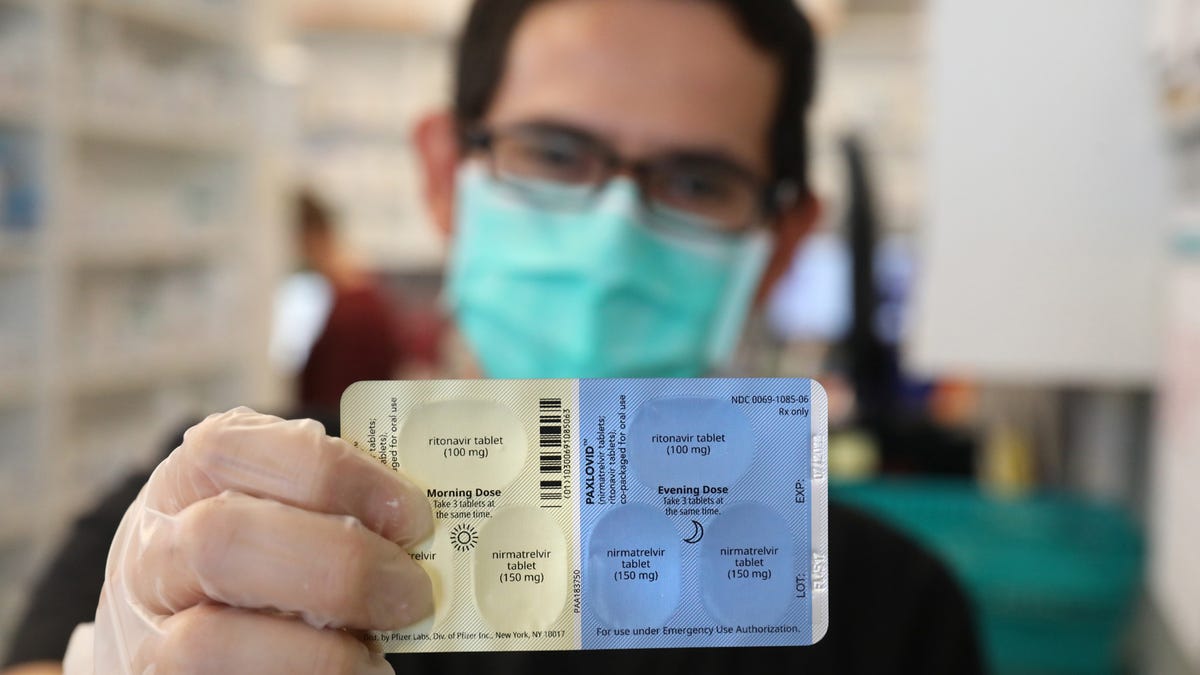
Paxlovid is a two-pill antiviral regimen from Pfizer that decreases the risk of hospitalization from COVID-19 by nearly 90%.
The White House is increasing the availability of Paxlovid, an antiviral medication authorized by the US Federal Drug Administration to treat COVID-19. The Biden administration said Tuesday it had purchased 20 million courses of the Pfizer-produced drug, more than any other country in the world.
The FDA in December gave Paxlovid the green light for those 12 and older who tested positive for COVID-19 and were at a higher risk for serious symptoms. That group includes older patients and those with cardiovascular disease, obesity and autoimmune diseases like HIV and diabetes.
But while research has shown Paxlovid can reduce the risk of hospitalization by nearly 90%, its uptake has been surprisingly slow.
Initially, the stock of the drug was limited but, according to a statement from the White House, the administration has worked with Pfizer and there's currently an "ample supply."
Still, many pharmacies say they haven't gotten the drug and some physicians are still confused about who should get a prescription.
"We've got to turn those pills into prescriptions and into things that patients can get so they can get better if they get infected," White House COVID coordinator Dr. Ashish Jha told NPR on Monday.
Here's what you need to know about Paxlovid, including how it works, where you can get it and why more people aren't using it.
What is Paxlovid?
Vaccines have proven effective at preventing COVID-19 and lessening the severity of the disease in so-called breakthrough cases. But for those already infected, antiviral drugs are needed to actively treat the condition.
Paxlovid is a combination of two medications: the antiviral ritonavir, paired with the newer drug nirmatrelvir. Nirmatrelvir is designed to block the activity of the SARS-CoV-2-3CL protease, an enzyme that the coronavirus needs to replicate. The ritonavir allows the medication to remain active in the body longer and at higher concentrations.
The FDA approved Paxlovid for COVID patients at a higher risk for serious illness, a group that includes those with cardiovascular disease, obesity and autoimmune conditions like diabetes.
The two work together as a protease inhibitor, disrupting the replication of SARS-CoV-2 in infected patients. Patients must take two tablets of nirmatrelvir and one tablet of ritonavir, twice a day for five days.
Pfizer says the pill has proven effective against more serious delta and the omicron variants of COVID-19. But it must be taken early to be effective -- within five days of symptoms manifesting.
Why hasn't it been prescribed to more people?
The FDA hasn't authorized Paxlovid for everyone. To qualify, you need to be considered part of a high-risk category. That means people with compromised immune systems or underlying conditions like cancer, heart disease, diabetes or obesity, as well as those 65 and older.
Even if you qualify, someone will still have to prescribe the drug, which means the pharmacy you get tested at will need to have a clinic, like CVS' MinuteClinic, where a professional can screen, diagnose and prescribe. Only 10% of CVS drugstores and even fewer Walgreens have clinicians on site.
When Paxlovid was first authorized, the highly contagious omicron variant also meant demand was far outstripping the supply of antivirals.
Dr. Elise Choi, Massachusetts chapter governor of the American College of Physicians, told the American Medical Association in early February that access was "being directed by the state health departments to specific local health departments, pharmacies, clinics, hospitals and physician offices."
She added that not all doctors could get the antivirals for their patients on demand. "There's a lot of state-to-state variability," Choi said.
Now that the supply of Paxlovid is sufficient, the Biden administration is focused on increasing availability and use.
Supply issues with Paxlovid have been addressed, White House medical adviser Dr. Anthony Fauci told MSNBC on April 13, but the drug "is being underutilized."
In Michigan, thousands of courses of Paxlovid and Merck's antiviral, Molnupiravir, have sat unused on pharmacy shelves, Dr. Natasha Bagdasarian, chief medical executive for Michigan, told NBC News.
Some doctors feel uncomfortable prescribing such a new drug, Bagdasarian said. Others worry about contraindications with medications for other conditions. Or they question whether a patient really qualifies as "high risk."
Some may not even realize Paxlovid is much more available than it was initially.
"What we need to do a better job of is getting the practicing physician and healthcare providers on the outside the knowledge and realization that this is an available intervention," Fauci told MSNBC.
Where can I get Paxlovid?
Walgreens and other pharmacy chains are offering COVID testing and antivirals through the White House's Test to Treat initiative.
Currently, there are only about 2,200 Test to Treat sites in pharmacies and other clinical settings, the White House said. But the federal government is working to ramp up that number.
"These sites will be targeted to meet demand and increase equitable access to lifesaving COVID-19 treatments and will function in direct collaboration with state and local health agencies," the administration said in its release.
And Paxlovid is being distributed directly to clinics, community health centers, long-term care facilities and veteran health centers.
The White House's COVID.gov website also shows locations where Paxlovid is available, as well as sites providing testing, vaccines and more.
How the White House is improving uptake of COVID antivirals
In addition to getting more Paxlovid produced, the new push by the White House aims to double the number of places antivirals are available to 40,000 locations in the next few weeks.
Tens of thousands of pharmacies nationwide can now order them for free directly from the federal government, and efforts to educate the public and health care providers about Paxlovid are also being ramped up.
President Joe Biden first announced the Test to Treat program, which allows people to get tested for COVID at their local pharmacy and receive free antivirals on the spot, at his State of the Union address March 1.
Pfizer was expected to deliver 1 million pills to drugstores by the end of March and "more than double that" in April, Biden said in his address.
Are there other approved oral antiviral medications?
Molnupiravir, developed by Merck and Ridgeback Biotherapeutics, received emergency authorization by the FDA in November, and the White House has purchased at least 1.7 million courses of the drug.
Molnupiravir's structure actually resembles the chemical-building blocks used to make COVID's RNA: The drug "sneaks" into the virus' RNA as it's being synthesized and mutates it to the point that the viral proteins it creates can no longer function.
Molnupiravir has proven less effective in preventing hospitalization and death than Paxlovid.
But Molnupiravir has ultimately proven much less effective than Paxlovid. (It was only narrowly approved by the FDA's advisory committee by 13 to 10.) In clinical trials, it only decreased the risk of hospitalization from COVID-19 by 30%, down from 50% in early results.
"That's not all that good. It's pretty lackluster," Katherine Seley-Radtke, a University of Maryland medicinal chemist told the journal Nature.
Will widespread use of antivirals lead to drug-resistant strains of COVID-19?
The overuse of some antibiotics has led to drug-resistant strains of diseases like tuberculosis and gonorrhea. While antivirals are different from antibiotics, the main reason that's unlikely to happen with Paxlovid is that the course of treatment is so short -- just five days.
"It won't put selective pressure on the virus to evolve," Monica Gandhi, an infectious disease expert at the University of California, San Francisco, told CNET earlier.
Will making antiviral treatments cause vaccination rates to decline?
"During this pandemic, we've done everything in our power to get people to take the vaccine -- we've incentivized, cajoled, mandated," Gandhi said.
"At this point, a year since the first vaccine was announced, I don't think we're going to change someone's mind."

