Hep C test for walking blood banks
A new hepatitis C test is on the way for prescreening blood donors, and it's showing some promise.
A new test to screen blood donors for hepatitis C (HCV) is showing promise, having scored the highest against five other systems during an evaluation by Walter Reed Army Institute of Research, according to developer OraSure Technologies.
When there's a shortage of blood for transfusions on the battlefield, medics turn to the Walking Blood Bank, i.e. any available soldier. However, short of prescreening every potential donor or using other time-consuming methods, there has been no way to be sure that a donor is disease free (PDF).
The company already offers a test for HIV, and now it looks ready to wrap up the HCV market. The test, known as OraQuick, reportedly delivered results "approximately three days sooner than available laboratory-based enzyme immunoassays and approximately 16 days earlier than the next most sensitive rapid HCV test."
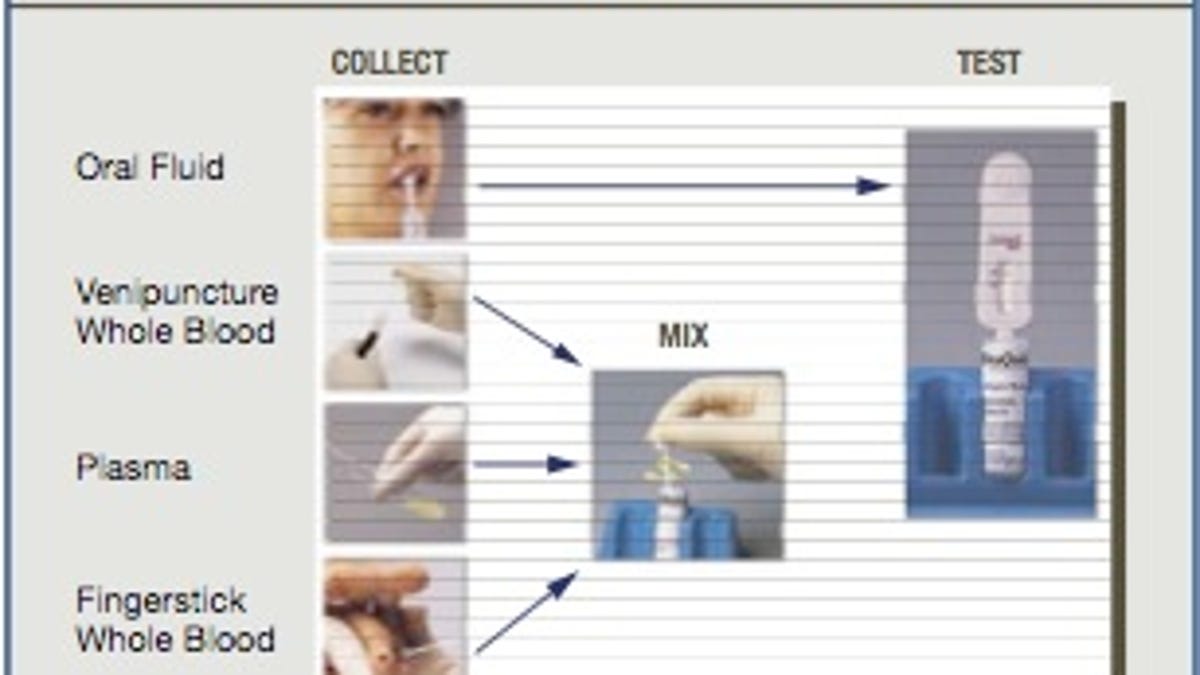
The evaluations used both plasma and blood specimens and even proved effective with new infections. "Early detection of seroconversion is an important measure of the sensitivity of a test and means that hepatitis C infection can be identified even with relatively recent exposure," boasts OraSure Technologies.
Hepatitis C can cause cirrhosis, liver failure, and liver cancer and affects approximately 4 million people in the U.S. alone, most of whom do not even know they're infected, according to the Centers for Disease Control and Prevention. Internationally, there are approximately 3 million to 4 million new HCV infections each year, making it one of the world's fastest-growing diseases, according to some estimates.
The product is undergoing clinical trials to obtain Food and Drug Administration approval for the test utilizing oral fluid, finger-stick and venous whole blood, plasma and serum specimen types, according to a company press release.